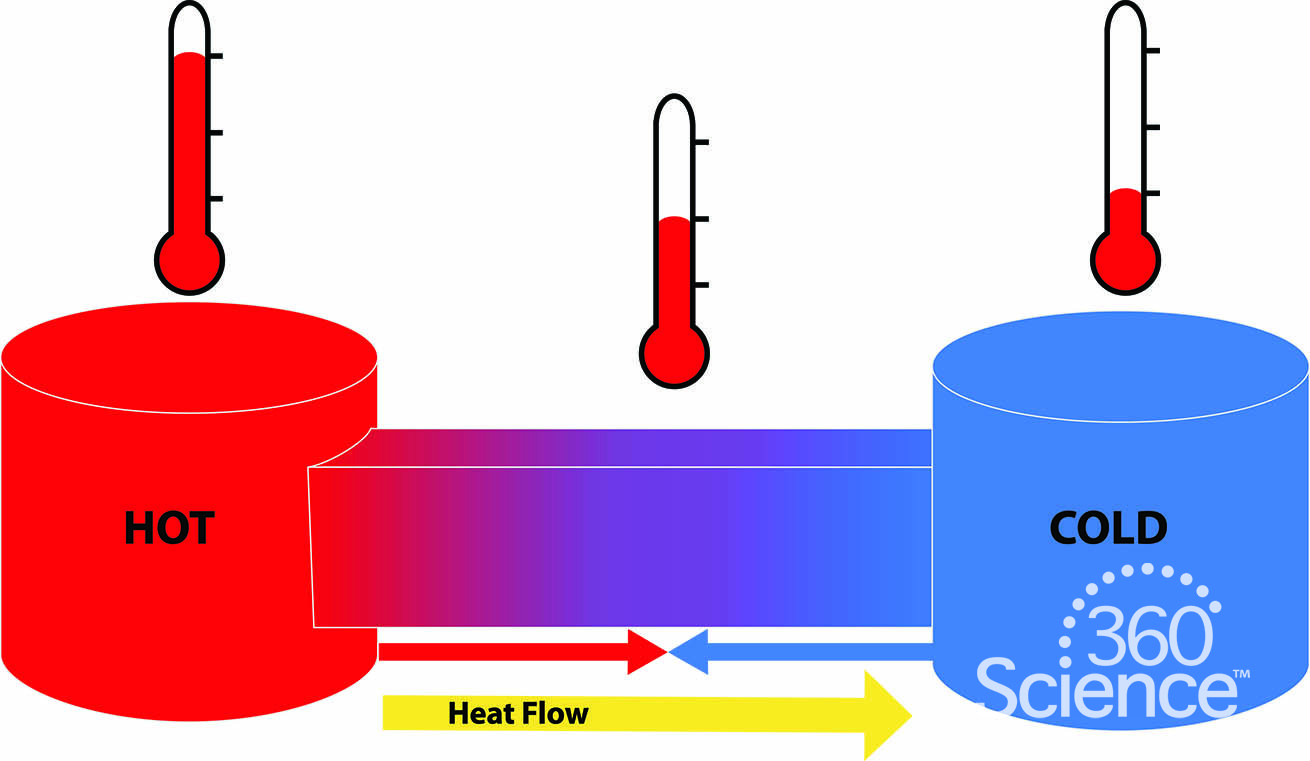
What Is The Difference Between Thermal Energy And Heat Quizlet . without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average kinetic. explain the difference between kinetic energy and potential energy. Define heat and work , and describe an. Thermal energy is the total kinetic and potential energy of all the particles in an. what is the difference between thermal energy and heat? when thermal energy flows, we refer to it as heat energy. Thermal energy is the total energy of all of the particles of a substance. what is the difference between thermal energy and heat? thermal energy of an object depends on the number of the particles in the object, the temperature and the arrangement the object. Temperature and heat are not the same thing because: thermal energy is the most basic form of energy that is responsible for random movements of the molecules within an object or a system. heat is the thermal energy transfer between systems or bodies due to a temperature difference. Thermal energy, in turn, is the kinetic energy of. Define chemical energy and thermal energy.
from www.flinnsci.com
what is the difference between thermal energy and heat? when thermal energy flows, we refer to it as heat energy. Thermal energy, in turn, is the kinetic energy of. Thermal energy is the total kinetic and potential energy of all the particles in an. thermal energy of an object depends on the number of the particles in the object, the temperature and the arrangement the object. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average kinetic. what is the difference between thermal energy and heat? explain the difference between kinetic energy and potential energy. Define heat and work , and describe an. Temperature and heat are not the same thing because:
360 Science Thermal Energy and Heat Transfer
What Is The Difference Between Thermal Energy And Heat Quizlet when thermal energy flows, we refer to it as heat energy. what is the difference between thermal energy and heat? Define chemical energy and thermal energy. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average kinetic. Define heat and work , and describe an. Thermal energy, in turn, is the kinetic energy of. explain the difference between kinetic energy and potential energy. Thermal energy is the total kinetic and potential energy of all the particles in an. heat is the thermal energy transfer between systems or bodies due to a temperature difference. thermal energy is the most basic form of energy that is responsible for random movements of the molecules within an object or a system. Temperature and heat are not the same thing because: what is the difference between thermal energy and heat? Thermal energy is the total energy of all of the particles of a substance. thermal energy of an object depends on the number of the particles in the object, the temperature and the arrangement the object. when thermal energy flows, we refer to it as heat energy.
From gohwanzhen-phyiscproject2009.blogspot.com
physic project 2009 Thermal energy What Is The Difference Between Thermal Energy And Heat Quizlet without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average kinetic. what is the difference between thermal energy and heat? explain the difference between kinetic energy and potential energy. Thermal energy, in turn, is the kinetic energy of. Define chemical energy and thermal energy. Thermal energy is the total. What Is The Difference Between Thermal Energy And Heat Quizlet.
From renewabletechy.com
What Is The Difference Between Thermal Energy And Heat? Renewable Tech What Is The Difference Between Thermal Energy And Heat Quizlet what is the difference between thermal energy and heat? thermal energy is the most basic form of energy that is responsible for random movements of the molecules within an object or a system. Temperature and heat are not the same thing because: heat is the thermal energy transfer between systems or bodies due to a temperature difference.. What Is The Difference Between Thermal Energy And Heat Quizlet.
From ask.modifiyegaraj.com
What Is The Difference Between Temperature And Thermal Energy Asking List What Is The Difference Between Thermal Energy And Heat Quizlet Thermal energy is the total energy of all of the particles of a substance. thermal energy of an object depends on the number of the particles in the object, the temperature and the arrangement the object. Thermal energy, in turn, is the kinetic energy of. without going into mathematical detail, we can say that thermal energy —the energy. What Is The Difference Between Thermal Energy And Heat Quizlet.
From www.tessshebaylo.com
What Is The Equation To Solve For Amount Of Heat Energy Tessshebaylo What Is The Difference Between Thermal Energy And Heat Quizlet heat is the thermal energy transfer between systems or bodies due to a temperature difference. Temperature and heat are not the same thing because: thermal energy of an object depends on the number of the particles in the object, the temperature and the arrangement the object. thermal energy is the most basic form of energy that is. What Is The Difference Between Thermal Energy And Heat Quizlet.
From studylib.net
Temperature vs. Thermal Energy What Is The Difference Between Thermal Energy And Heat Quizlet Temperature and heat are not the same thing because: what is the difference between thermal energy and heat? Define chemical energy and thermal energy. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average kinetic. thermal energy of an object depends on the number of the particles in the. What Is The Difference Between Thermal Energy And Heat Quizlet.
From www.difference.minaprem.com
Difference Between Heat and Temperature What Is The Difference Between Thermal Energy And Heat Quizlet Temperature and heat are not the same thing because: heat is the thermal energy transfer between systems or bodies due to a temperature difference. explain the difference between kinetic energy and potential energy. Thermal energy is the total kinetic and potential energy of all the particles in an. Thermal energy, in turn, is the kinetic energy of. . What Is The Difference Between Thermal Energy And Heat Quizlet.
From www.albert.io
What's the Difference Between Temperature and Heat? Albert.io What Is The Difference Between Thermal Energy And Heat Quizlet thermal energy of an object depends on the number of the particles in the object, the temperature and the arrangement the object. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average kinetic. Thermal energy is the total energy of all of the particles of a substance. Define chemical energy. What Is The Difference Between Thermal Energy And Heat Quizlet.
From www.vrogue.co
Difference Between Heat And Temperature With Comparis vrogue.co What Is The Difference Between Thermal Energy And Heat Quizlet Define chemical energy and thermal energy. what is the difference between thermal energy and heat? what is the difference between thermal energy and heat? heat is the thermal energy transfer between systems or bodies due to a temperature difference. explain the difference between kinetic energy and potential energy. thermal energy of an object depends on. What Is The Difference Between Thermal Energy And Heat Quizlet.
From brainly.com
What is the difference between thermal energy and heat? What Is The Difference Between Thermal Energy And Heat Quizlet Temperature and heat are not the same thing because: when thermal energy flows, we refer to it as heat energy. heat is the thermal energy transfer between systems or bodies due to a temperature difference. Thermal energy, in turn, is the kinetic energy of. without going into mathematical detail, we can say that thermal energy —the energy. What Is The Difference Between Thermal Energy And Heat Quizlet.
From quizlet.com
Thermal Energy Diagram Quizlet What Is The Difference Between Thermal Energy And Heat Quizlet thermal energy of an object depends on the number of the particles in the object, the temperature and the arrangement the object. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average kinetic. Temperature and heat are not the same thing because: thermal energy is the most basic form. What Is The Difference Between Thermal Energy And Heat Quizlet.
From modernize.com
How Does Thermal Energy Work? Modernize What Is The Difference Between Thermal Energy And Heat Quizlet thermal energy is the most basic form of energy that is responsible for random movements of the molecules within an object or a system. what is the difference between thermal energy and heat? Temperature and heat are not the same thing because: when thermal energy flows, we refer to it as heat energy. thermal energy of. What Is The Difference Between Thermal Energy And Heat Quizlet.
From quizlet.com
Heat Transfer Diagram Quizlet What Is The Difference Between Thermal Energy And Heat Quizlet Thermal energy is the total kinetic and potential energy of all the particles in an. when thermal energy flows, we refer to it as heat energy. Temperature and heat are not the same thing because: what is the difference between thermal energy and heat? thermal energy of an object depends on the number of the particles in. What Is The Difference Between Thermal Energy And Heat Quizlet.
From www.slideshare.net
HEATING AND COOLING (TEMPERATURE AND THERMAL ENERGY) LESSON ONE What Is The Difference Between Thermal Energy And Heat Quizlet Define heat and work , and describe an. thermal energy of an object depends on the number of the particles in the object, the temperature and the arrangement the object. Thermal energy is the total kinetic and potential energy of all the particles in an. Thermal energy, in turn, is the kinetic energy of. Temperature and heat are not. What Is The Difference Between Thermal Energy And Heat Quizlet.
From studentcomfort26.gitlab.io
Spectacular What's An Example Of Thermal Energy Alternating Current What Is The Difference Between Thermal Energy And Heat Quizlet Temperature and heat are not the same thing because: Thermal energy is the total energy of all of the particles of a substance. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average kinetic. what is the difference between thermal energy and heat? thermal energy of an object depends. What Is The Difference Between Thermal Energy And Heat Quizlet.
From www.slideshare.net
Chapter 12 Thermal Energy What Is The Difference Between Thermal Energy And Heat Quizlet Define chemical energy and thermal energy. Temperature and heat are not the same thing because: Thermal energy, in turn, is the kinetic energy of. explain the difference between kinetic energy and potential energy. thermal energy is the most basic form of energy that is responsible for random movements of the molecules within an object or a system. Define. What Is The Difference Between Thermal Energy And Heat Quizlet.
From tay-ja.blogspot.com
What Is Thermal Energy 5th grade chapter 14 section 4 what is What Is The Difference Between Thermal Energy And Heat Quizlet Thermal energy is the total kinetic and potential energy of all the particles in an. when thermal energy flows, we refer to it as heat energy. explain the difference between kinetic energy and potential energy. thermal energy of an object depends on the number of the particles in the object, the temperature and the arrangement the object.. What Is The Difference Between Thermal Energy And Heat Quizlet.
From studyonline.netlify.app
Similarities between thermal energy and temperature What Is The Difference Between Thermal Energy And Heat Quizlet Thermal energy is the total energy of all of the particles of a substance. when thermal energy flows, we refer to it as heat energy. thermal energy is the most basic form of energy that is responsible for random movements of the molecules within an object or a system. Define chemical energy and thermal energy. what is. What Is The Difference Between Thermal Energy And Heat Quizlet.
From www.biocab.org
Heat and Thermal Energy What Is The Difference Between Thermal Energy And Heat Quizlet heat is the thermal energy transfer between systems or bodies due to a temperature difference. when thermal energy flows, we refer to it as heat energy. thermal energy is the most basic form of energy that is responsible for random movements of the molecules within an object or a system. Temperature and heat are not the same. What Is The Difference Between Thermal Energy And Heat Quizlet.